三硫丙酮
外观
(重定向自三聚硫代丙酮)
| 三硫丙酮 | |
|---|---|

| |
| IUPAC名 Hexamethyl-1,3,5-trithiane 六甲基-1,3,5-三噻烷 | |
| 别名 | 三硫代丙酮 三聚硫代丙酮 trithioacetone |
| 识别 | |
| CAS号 | 828-26-2 |
| PubChem | 13233 |
| ChemSpider | 12678 |
| SMILES |
|
| Beilstein | 5-19-09-00119 |
| 性质 | |
| 化学式 | C9H18S3 |
| 摩尔质量 | 222.43 g·mol−1 |
| 密度 | 1.0660-1.0700 g/mL[1] |
| 熔点 | 21.8℃[2] |
| 沸点 | 107℃(10 mmHg)[1] |
| 折光度n D |
1.5390-1.5430[1] |
| 危险性 | |
GHS危险性符号
| |
| GHS提示词 | 警告 |
| H-术语 | H315, H319, H335 |
| P-术语 | P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
三硫丙酮(2,2,4,4,6,6-六甲基-1,3,5-三噻烷),也称三聚硫代丙酮,是一种有机物,化学式为C
9H
18S
3,共价结构式为[–C(CH
3)
2–S–]
3。该化合物可以被看作是1,3,5-三硫雜環己烷的衍生物,即一个碳原子和硫原子交替出现的六元环,每个碳再连接两个甲基,由甲基取代所有的氢原子[3][1]。
该化合物是硫代丙酮(丙烷-2-硫酮)的稳定的环状三聚物,而硫代丙酮本身是一个不稳定的化合物[4][5]。相比之下,与之类似的化合物三丙酮(2,2,4,4,6,6-六甲基-1,3,5-三噁烷,氧原子代替硫原子)是不稳定的,而单体丙酮(2-丙酮)本身是稳定的。
制备
[编辑]三硫丙酮最早由欧根·鲍曼(Eugen Baumann)和E·弗罗姆(E. Fromm)于1889年通过硫化氢与丙酮的反应而合成[5]。在25℃、酸性、有ZnCl
2催化剂的条件下,得到的产物是60%-70%的三硫丙酮,30%-40%的2,2-丙二硫醇,以及少量的两种异构体杂质3,3,5,5,6,6-六甲基-1,2,4-三硫雜環己烷和4-巯基-2,2,4,6,6-五甲基-1,3-二硫雜環己烷[5]。该产品也可以通过烯丙基异丙基硫醚(3-异丙硫基丙-1-烯)的热解来获得[6][7]。
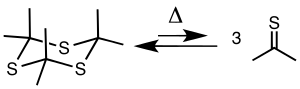
反应
[编辑]三硫丙酮在500-650℃和5-20 mmHg下热解产生硫丙酮,可通过−78℃的冷阱收集。
用途
[编辑]三硫丙酮存在于一些香料中。其FEMA编号为3475。[8][9][10][11]
毒性
[编辑]参见
[编辑]- 2,4,6-三甲基-1,3,5-三噻烷
- 六甲基环三硅氧烷,硅氧杂环之相似物
- 六甲基环三硅氮烷,硅氮杂环之相似物[12]
- 2,2,4,4,6,6-六甲基-1,3,5-三硒-2,4,6-三锡环己烷,锡-硒杂环之相似物[13][14]
参考资料
[编辑]- ^ 1.0 1.1 1.2 1.3 TCI America (2020): "Product H1278: 2,2,4,4,6,6-Hexamethyl-1,3,5-trithiane (页面存档备份,存于互联网档案馆)". Online catalog page, accessed on 2020-01-01.
- ^ 2.0 2.1 NCBI PubChem (2010): "2,2,4,4,6,6-Hexamethyl-1,3,5-trithiane (页面存档备份,存于互联网档案馆)". Online chemical data sheet, accessed on 2020-01-01.
- ^ David S. Breslow, Herman Skolnik (2009): Multi-Sulfur and Sulfur and Oxygen Five- and Six-Membered Heterocycles, Part 2; page 712. Volume 68 of Chemistry of Heterocyclic Compounds. ISBN 9780470188330
- ^ R. D. Lipscomb and W. H. Sharkey (1970): "Characterization and polymerization of thioacetone". Journal of Polymer Science - Part A: Polymer Chemistry, volume 8, issue 8, pages 2187–2196. doi:10.1002/pol.1970.150080826
- ^ 5.0 5.1 5.2 William H. Sharkey (1979): "Polymerization through the carbon-sulfur double bond". Polymerization, series Advances in Polymer Science, volume 17, pages 73-103. doi:10.1007/3-540-07111-3_2
- ^ William J. Bailey and Hilda Chu (1965): "Synthesis of polythioacetone". ACS Polymer Preprints, volume 6, pages=145–155
- ^ Horst Bohme, Hans Pfeifer, and Erich Schneider (1942): "Dimeric thioketones". Berichte der Deutschen Chemischen Gesellschaft, volume 75B, issue 7, pages 900–909. doi:10.1002/cber.19420750722 Note: This early report mistakes the trimer for the monomer.
- ^ 8.0 8.1 E. J. Moran, O. D. Easterday, and B. L. Oser (1980): "Acute oral toxicity of selected flavor chemicals". Drug and Chemical Toxicology, volume 3,issue 3, pages 249-258.PMID 7449655 doi:10.3109/01480548009002221
- ^ World Health Organization (1999): "Trithioacetone[失效連結]". Online data sheet in the Evaluation of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Accessedd on 2020-01-02.
- ^ G. Ohloff and I. Flament (1979): "The Role of Heteroatomic Substances in the Aroma Compounds of Foodstuffs". In Fortschritte der Chemie Organischer Naturstoffe (Progress in the Chemistry of Organic Natural Products), volume 36, pages 231-283. doi:10.1007/978-3-7091-3265-4_2
- ^ EUR-Lex (2012): "Table entry 15.009: Trithioacetone (页面存档备份,存于互联网档案馆)". In EU Regulation No. 872/2012, Document 32012R0872, Official Journal of the EU - Series L, volume 267, pages 1–161.
- ^ Stuart D. Brewer and Charles P. Haber (1948): "Alkylsilazanes and Some Related Compounds". Journal of the American Chemical Society, volume 70, issue 11, pages 3888-3891. doi:10.1021/ja01191a106
- ^ B. M. Mikhova (2008), "NMR Data for Carbon-13 - C6H18Se3Sn3" in Landolt-Börnstein - Group III Condensed Matter, volume 35 Nuclear Magnetic Resonance Data, subvolume D5, Organometallic Compounds. doi:10.1007/978-3-540-74189-3_1362
- ^ Martin Dräger, Axel Blecher, Hans-Jürgen Jacobsen, Bernt Krebs (1978): "Molekül- und kristallstruktur von hexamethylcyclo-tristannaselenan [(CH
3)
2SnSe]
3". Journal of Organometallic Chemistry, volume 161, issue 3, pages 319-325. doi:10.1016/S0022-328X(00)92243-5
