類器官
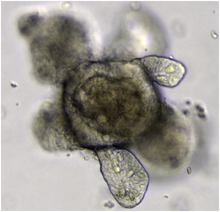
類器官(英語:Organoid)是體外培養生成的立體細胞團,是特定器官的迷你簡化版本,模仿該器官的關鍵功能、結構和生物複雜性。[1]類器官的培養可以起源於胚胎幹細胞或者成體幹細胞等多能性幹細胞、人工誘導性多能幹細胞以及癌症幹細胞,這些細胞的自我更新以及分化潛能賦予其在立體培養條件下中自組裝的能力。類器官的發展提供科學家與工程師在實驗室中研究疾病與藥物開發的簡化模型。[2]協助個人化醫療、基因和細胞療法、組織工程和再生醫學等領域的發展。[3]
歷史
[編輯]體外培養器官的始於一個解離再聚集實驗[4],科學家亨利·範·彼特斯·威森發現透過機械方式打散的海綿細胞可以自發性地重新聚集並組裝成完整個體[5]。在隨後的幾十年中,多個實驗室成功於兩棲動物[6]和雞胚胎[7]身上取得的器官組織重現解離後自組裝的實驗,在體外生成各類型的器官[4]。1975年,科學家透過共培養角質形成細胞和3T3纖維母細胞,首次在體外觀察到第一個組織樣細胞群的形成[8]。這些透過機械外力打散器官後的細胞再聚集與自組裝的現象促致馬爾科姆·斯坦伯格提出了差異黏附假說(Differential adhesion hypothesis,DAH)[4]。隨著幹細胞生物學的出現,科學家開始認識到幹細胞在體外形成器官的潛力,因為觀察到當其形成畸胎瘤或擬胚體時,分化的細胞可以組織成類似於在體內發現的各種組織類型[4]。類器官的出現始於細胞培養從二維平面基質轉為三維立體基質的階段,隨著細胞外基質的發展,3D培養基方法的方法成為可能[9],以允許器官立體結構的發育。[4]20世紀80年代末,米娜·貝塞爾及其同事證明,富含層粘連蛋白的凝膠可用作乳腺上皮細胞培養分化的基底膜[10][11]。如今,各類器官的培養方法已被提出並逐漸成熟[12]。在20世紀90年代,除了ECM提供細胞生長物理性質上的支持被提出外,還報導了ECM內的成分透過與基於整合素的黏著蛋白通路相互作用而影響基因表現[13]。2006年,Yaakov Nahmias和David Odde展示了血管肝類器官的自組裝在體外環境維持了50多天[14]。2008年,日本理化學研究所的Yoshiki Sasai和他的團隊證明,幹細胞可以被誘導成神經細胞球並且自組織成獨特的層狀構造[15]。2009年,荷蘭皇家藝術與科學研究所和烏特勒支大學的Hans Clevers實驗室表明,單個表達LGR5的腸幹細胞可以在體外自組織成隱窩絨毛結構並且無須提供間質區位,這使它們成為第一個類器官[16]。2010年,Mathieu Unbekandt和Jamie A. Davies證明了利用鼠胚衍生的腎幹細胞可產生腎類器官[17]。2014年,王峮及其同事設計了基於I型膠原和層粘連蛋白的凝膠和合成泡沫生物材料,用於培養和運輸腸道類器官[18],並將DNA功能化的金納米顆粒封裝到腸道類器官中,成為可供藥物運送與基因治療的腸道特洛伊木馬(intestinal Trojan horse)[19]。後續的研究顯示這些類器官在體外[20]和體內同樣具有顯着的生理功能[21][22]。
其他重大的早期進展包括2013年,奧地利科學院分子生物技術研究所的Madeline Lancaster制定了一項流程,可以從多能幹細胞開始生成模仿人類大腦細胞組織發育的大腦類器官[23]。荷蘭皇家藝術與科學研究所和烏特勒支大學醫學中心的Meritxell Huch與Craig Dorrell證明,來自受損小鼠肝臟的單個Lgr5+細胞可以在基於Rspo1的培養基中複製並擴增數個月並最終形成肝類器官[24]。2014年,伊利諾大學厄巴納-香檳分校的Artem Shkumatov等人證明,通過調控胚胎幹細胞粘附的基質硬度,可以形成心血管類器官。生理上的硬度特性促進了EB的立體性質與心肌分化[25]。2015年,Takebe等人通過將多能幹細胞衍生的組織特異性祖細胞或相關組織樣本與內皮細胞和間質幹細胞相結合,展示了一種從不同組織形成器官芽的通用方法。他們認為,通過自組織凝聚原理產生的不太成熟的組織或器官芽可能是移植後重建成熟器官功能的最有效方法,而不是由發育上更加成熟階段的細胞凝聚物[26]。
特性
[編輯]Lancaster和Knoblich[4]將類器官定義為從幹細胞或器官前驅細胞發育而來的器官特異性細胞類型的集合,透過細胞分選和空間限制的譜系定型以類似於體內的方式進行自組織,並表現出以下特徵特性:
培養過程
[編輯]類器官的生長通常需要在立體培養基中培養幹細胞或祖細胞[4]。幹細胞具有自我更新和分化成各種細胞類型的能力,並且能夠用於了解發育和疾病進展的過程[27]。因此,源自幹細胞的類器官能夠在器官水平上研究生理學[28]。立體培養基可以使用細胞外基質水凝膠(例如Matrigel或Cultrex BME)製成,這是一種富含層粘連蛋白的細胞外基質,由Engelbreth-Holm-Swarm腫瘤細胞株分泌[29],可以通過將幹細胞嵌入基質中來製備類器官[4]。當多能性幹細胞用於創建類器官時,細胞通常(但並非總是)形成擬胚體[4]。然後用模式因子對這些擬胚體進行處理,以驅動所需類器官特徵的形成[4]。此外,也可以使用目標器官中提取的成體幹細胞創建類器官,並在立體培養基中培養[30]。
生物化學的特性已被納入類器官培養中,藉由添加形態發生素、形態發生抑制劑或生長因子,可以誘導胚胎幹細胞或成體幹細胞發育成為類器官。血管化技術可用於賦予微環境在生理上接近其相對應部位的特性。可以藉由微流體系統、血管內皮生長因子輸送系統和內皮細胞塗層模塊來達成可促進氧氣或營養物質進入類器官內部的血管系統[9]。利用源自患者的誘導多能幹細胞(iPSC)[31]和基於CRISPR/Cas9的基因編輯技術[32],可以生成基因組編輯或突變的多能幹細胞(PSCs),並改變信號傳遞特性,以控制器官模型內的內在性質。
類型
[編輯]使用類器官可以概括多種器官結構[4]。本節旨在透過提供一份精簡的器官模型清單,概述目前該領域的現狀,並根據最新文獻對每個器官模型進行簡要概述,並提供其在研究中的應用示例。
腦類器官
[編輯]腦類器官是指體外培養的類似於大腦的微型器官。大腦類器官於旋轉生物反應器在三維環境下中培養人類多能幹細胞產生,並需要數月的時間發育[23]。這對腦部發育、生理學和功能的研究中具有潛在的應用。腦類器官可能會對外部刺激產生「簡單的感覺」,神經科學家也對這些器官可能發展出感知能力表示擔憂。他們提出,該技術的進一步發展需要受到嚴格的監督[33][34][35]。2023年,研究人員建造了一台混合生物計算機,將實驗室培養的人腦類器官與傳統電路相結合,可以完成語音識別等任務[36]。腦類器官目前正用於研究和開發類器官智能(OI)技術[37]。
胃腸道類器官
[編輯]胃腸道類器官是指概括胃腸道結構的類器官。胃腸道起源於內胚層,在發育過程中形成一個管狀構造,可以分為三個不同的區域,與其他器官一起產生胃腸道的以下部分:[4]
胃腸道類器官又可細分為以下數種:
腸類器官
[編輯]迄今為止,腸類器官[16]屬於直接由腸組織或多能幹細胞產生的腸道類器官[4]。促使人類多能幹細胞形成腸類器官的方法是,首先使用激活素A驅動細胞進入中內胚層狀態,然後對Wnt3a和Fgf4信號通路進行上調,因為它們已被證明可以促進組織走向後腸道細胞命運[4]。腸類器官也可以由腸幹細胞產生,從成體組織中提取並在立體培養基中培養[30]。這些成體幹細胞衍生的類器官通常被稱為腸類器官或類結腸類器官,具體取決於它們的起源部分,並且是從人類和小鼠腸道中建立的[16][38][39]。腸類器官由圍繞中央管腔的單層極化腸上皮細胞組成。因此,通過概括腸道的功能、生理學和組織,並維持結構中正常存在的所有細胞類型(包括腸幹細胞),概括腸道的隱窩絨毛結構[4]。因此,腸類器官是研究腸道營養轉運[40][41]、藥物吸收和遞送[42][43]、納米材料和納米醫學[44][45]、腸泌素分泌[46][47]和各種腸道病原體感染[48][49]等議題的有力模型。
例如,王峮團隊利用腸類器官衍生的粘膜模型設計了人工病毒納米顆粒作為口服藥物遞送載體(ODDV)[50],並展示了利用新建立的結腸類器官作為高通量藥物篩選、毒性分析工具的新概念。測試和口服藥物開發[51]。腸類器官還以如此高的保真度再現了隱窩絨毛結構,以至於它們已成功移植到小鼠腸道中,因此被高度視為有價值的研究模型[4]。腸類器官已被利用的研究領域之一是幹細胞生態位。腸類器官被用來研究腸幹細胞區位的性質,並證明了IL-22在維持腸幹細胞中的重要作用[52]以及其他細胞類型(如神經元和成纖維細胞)在維持腸道幹細胞的重要性[30]。在感染生物學領域,人們已經探索各類基於腸道類器官的模型系統。一方面,只需將類器官與感興趣的腸道病原體混合即可大量感染[53]。然而,為了模擬更接近自然情況下,由腸腔開始的感染途徑,需要使用病原體進行顯微注射[54][55]。此外,腸類器官的極性可以反轉[56],甚至可以解離成單個細胞並以二維單層培養[57][58],以便使上皮的頂端和基底外側更容易接近。最後,腸類器官也顯示出用於治療的潛力[59]。

為了更準確地再現體內腸道,開發了腸道類器官和免疫細胞的共培養方式[58]。此外,器官晶片模型將腸道類器官與其他細胞或體內環境(例如內皮細胞、免疫細胞以及蠕動)結合起來[60][61]。
胃類器官
[編輯]胃類器官部分地概括了胃的生理性質。通過在三維培養條件下對FGF、WNT、BMP、視黃酸和EGF信號通路進行時間尺度上的調控,可以從多能幹細胞直接生成胃類器官[62]。胃類器官也可以由LGR5+的胃成體幹細胞產生[63]。胃類器官已被用作研究癌症[64][65]以及其他人類疾病發育的模型[62]。例如,一項研究[65]調查了患者轉移性腫瘤背後的潛在遺傳變化,發現相較於同一患者身上的原發性腫瘤,轉移性腫瘤的TGFBR2基因的兩個等位基因均發生突變。為了進一步評估TGFBR2在轉移中的作用,研究人員創建了TGFBR2基因敲落的類器官,透過這種類器官,他們證明TGFBR2活性降低會導致體內與體外環境下的惡性腫瘤侵襲與轉移。
舌類器官
[編輯]舌類器官是概括舌頭生理學各方面的類器官。在立體培養條件下,透過EGF、WNT和TGF-β的調控,使用表達BMI1的上皮幹細胞培養出上皮舌類器官[66]。然而,這種類器官培養物缺乏味覺受體[66]。相較之下,含有味覺細胞的味蕾類器官則是使用LGR5+或CD44+的輪狀乳突幹細胞/前驅細胞[67]或者Lgr5+或LGR6+的味覺幹細胞創建的[68]。
其他
[編輯]- 細胞排斥性微量滴定板的最新進展使得能夠快速、經濟高效地篩選大分子藥物(例如針對胰腺癌3D模型的庫)。這些模型在表型和表達譜上與David Tuveson博士實驗室發現的模型一致。
- 上皮類器官[16][80]
- 肺類器官[81]
- 腎類器官[17][82][83][84]
- 原腸胚(胚胎類器官)[85][86][87][88]——可以生成所有胚胎軸並且在前後軸的方向與Hox基因表達的形式共線[88]。
- 囊胚類器官[89][90][91]
- 子宮內膜類器官[92]
- 心臟類器官[93]——2018年,中空心臟類器官被提出,並表現心搏且可以對刺激產生反應而改變跳動速度[94]。
- 視網膜類器官[95][96]
- 乳腺癌類器官[97]
- 結直腸癌類器官[98]
- 膠質母細胞瘤類器官[99]
現今,來自患者的外植體(patient derived explants,PDX)或直接來自癌症組織的三維器官模型已經可以輕易製備,並且可將其用於現有核准藥物的高通量篩選。
由腦微血管內皮細胞(BMECs)、星形細胞和周細胞組成的自組裝細胞聚集體正逐漸成為物質穿膜和微流體模型的潛在替代方案。這些器官模型能夠生成血腦屏障(BBB)的許多特徵,如緊密連接的表達、分子運輸蛋白和藥物排出泵,因此可以用來模擬藥物穿越BBB的過程。此外,它們可以作為評估BBB與相鄰腦組織之間相互作用的模型,並提供了一個了解新藥物克服BBB的綜合能力以及其對腦組織的影響的平台。此類模型具有高度可擴展性,且比微流體裝置更容易製造和操作。然而,它們對於重建BBB的形態和生理學以及模擬生理流動和剪應力的能力有限[103]。
基礎研究
[編輯]類器官能夠協助研究細胞與細胞間、細胞與環境之間的相互作用以及疾病和藥物如何影響他們的作用。體外培養使該系統易於操作及監測。器官的實際體積過大使得物質滲透受到限制而不易培養,但類器官的小尺寸可以規避此問題。另一方面,類器官並不表現出所有器官特徵,並且與其他器官的相互作用在體外也無法重現。雖然腸道類器官的第一個研究方向是用於探討幹細胞特性的調控[16],但如今也用於研究營養物質的攝取、藥物轉運和腸泌素的分泌等議題[104]。這對於吸收不良疾病以及肥胖、胰島素抵抗和糖尿病等代謝疾病具有重要意義。
疾病模型
[編輯]類器官提供建立人類疾病細胞模型的機會,可以在實驗室中進行研究以更好地了解疾病的原因並確定可能的治療方法。類器官在這方面的潛力首次在小頭畸形的遺傳研究中顯現,其中患者細胞被用來製造腦類器官,這種類器官較小並且在早期神經元生成中表現出異常[23]。另一個案例是將CRISPR應用於人類多能幹細胞,在與兩種不同腎臟疾病(多囊腎病和局灶節段性腎小球硬化症)相關的基因中引入靶向突變[83]。這些經過CRISPR修飾的多能幹細胞隨後被培養成人類腎類器官,表現出疾病特異性表型。來自患有多囊腎病突變的幹細胞的腎臟類器官由腎小管形成了巨大的半透明囊腫結構。當在懸浮的情況下培養時,這些包囊的大小在數個月內達到直徑1厘米[105]。與局灶節段性腎小球硬化症相關的基因發生突變的腎類器官,其足細胞(該疾病中受影響的過濾細胞)之間出現了細胞連接的缺陷[106]。重要的是,這些疾病表型在具有相同遺傳背景但缺乏CRISPR突變的對照類器官中不存在[83][105][106]。將這些類器官表型與小鼠和人類的患病組織進行比較,發現它們與早期發育缺陷有相似之處[105][106]。
正如Takahashi和Yamanaka於2007年首次發表的那樣,誘導多能幹細胞(iPSC)也可以從患者皮膚纖維母細胞中重編程[107]。這些幹細胞攜帶患者的確切遺傳背景,包括可能導致人類疾病發展的任何基因突變。由於ORCL1突變而患有Lowe綜合徵的患者已將這些細胞分化為腎臟類器官[108]。該報告比較了患者iPSC與不相關的對照iPSC分化的腎類器官,並證明患者腎細胞無法調動高爾基體中的轉錄因子SIX2[108]。因為SIX2是腎單元前驅細胞的一個明確標記,作者得出結論為,洛氏綜合徵(近曲小管在吸收的整體衰竭或范康尼氏症候群)中常見的腎臟疾病可能與腎單元引起的改變有關,其中祖細胞缺乏這種重要的SIX2基因表達[108]。
其他研究使用CRISPR來修復患者iPSC細胞中的突變,以創建等位基因對照,該對照可以與iPSC重編程同時進行[109][110][111]。將患者iPSC衍生的類器官與同基因對照進行比較是該領域當前的黃金標準,因為它允許將感興趣的突變分離為實驗模型中的唯一變量[112]。在一份報告中,將源自IFT140複合雜合突變的Mainzer-Saldino綜合徵患者iPSC的腎類器官與等基因對照類器官進行比較,其中通過CRISPR修正了產生無活性mRNA轉錄物的IFT140突變體[110]。患者腎類器官表現出與現有動物模型一致的異常纖毛形態,在基因修正的類器官中將其恢復為野生型狀態[110]。比較患者和對照類器官中純化的上皮細胞的轉錄組突顯了涉及細胞極性、細胞-細胞連接和動力蛋白運動組裝的途徑,其中一些途徑與腎纖毛病表型家族中的其他基因型有關[110]。另一份利用等基因對照的報告表明,先天性腎病綜合徵患者產生的腎臟類器官的腎小球中去氧腎上腺素定位異常[111]。
最後,諸如上皮代謝之類的事情也可以利用類似方式建模[113]。
個人化醫療
[編輯]Clevers小組建立的方法可以從直腸活檢樣本中培養出的腸類器官,目前已被用於模擬囊腫性纖維化[114],並促使類器官首次運用於個人化醫療[115]。囊腫性纖維化是一種遺傳性疾病,由囊腫性纖維化穿膜傳導調節基因(Cystic fibrosis transmembrane conductance regulator,CFTR)的突變引起,該基因編碼位於健康上皮表面維持液體所需的離子通道。Jeffrey Beekman實驗室於2013年進行的研究描述,以毛喉素或霍亂毒素等cAMP升高激動劑刺激結直腸類器官,會以完全CFTR依賴性的方式誘導類器官快速腫脹[114]。非囊腫性纖維化患者的類器官因為液體輸送到類器官管腔而對毛喉素產生反應並膨脹,相較之下,來自囊腫性纖維化患者的類器官則嚴重減少或不存在。修復CFTR蛋白的療法可以恢復腫脹,這表明可以在臨床前實驗室環境中量化個體對CFTR調節療法的反應。2013年,Schwank等人於更進一步證明腸道囊腫性纖維化類器官的異常表型可以透過CRISPR-Cas9基因編輯進行修復[116]。
2016年,Dekkers等人的後續研究表明,來自囊腫性纖維化患者的腸道類器官之間由毛喉素誘導的腫脹程度差異與已知的診斷和預後標誌物(例如CFTR基因突變或CFTR功能的體內生物標記)相關[115]。此外,他們證明具有特定CFTR突變的腸道類器官接受CFTR調節劑處裡後的效果與已發表的臨床試驗結果相似。這促使臨床前研究發現來自具有極其罕見的CFTR突變且未經過治療的患者類器官對臨床使用的CFTR調節劑有強烈反應。這些臨床前類器官測試所得到的治療成效被後續Kors van der Ent所帶領的團隊執行的臨床試驗所證實。這些研究首次表明類器官可以應用於個人化醫療。
類器官移植
[編輯]2022 年,類器官被首次用於移植手術。一名患有潰瘍性結腸炎的患者,藉由採集其健康結腸黏膜的細胞進行體外培養1個月後,將這些細胞生長而成的類器官重新移植回患者身上,取得良好的治療效果[117][118]。
作為發育生物學的模型
[編輯]類器官為研究人員提供了研究發育生物學的模型[119]。自從多能幹細胞被提出後以來,利用二維培養在體外定向誘導多能幹細胞的分化已經取得了巨大的進展[119]。如今,多能性幹細胞培養的技術進步搭配3D培養技術的發展,使得培養各類器官內的特定細胞組織成為可能[119]。因此,這些類器官的使用極大地促進了我們對器官發生過程和發育生物學領域的理解[119]。例如,中樞神經系統的發育中,類器官的研究有助於科學家理解視神經盤形成過程中物理力量的扮演的角色[119][120]。近期的研究則專注於延長皮質類器官的生長週期並且取得顯著的進展。在一些研究中,類器官存在將近一年,並表現出人類胎兒發展階段的部分特徵[121]。
參見
[編輯]參考文獻
[編輯]
- ^ Zhao, Zixuan; Chen, Xinyi; Dowbaj, Anna M.; Sljukic, Aleksandra; Bratlie, Kaitlin; Lin, Luda; Fong, Eliza Li Shan; Balachander, Gowri Manohari; Chen, Zhaowei; Soragni, Alice; Huch, Meritxell. Organoids. Nature Reviews Methods Primers. 2022-12-01, 2 (1) [2024-02-05]. ISSN 2662-8449. PMC 10270325
 . PMID 37325195. doi:10.1038/s43586-022-00174-y. (原始內容存檔於2024-04-02) (英語).
. PMID 37325195. doi:10.1038/s43586-022-00174-y. (原始內容存檔於2024-04-02) (英語).
- ^ Mullard, Asher. Mini-organs attract big pharma. Nature Reviews Drug Discovery. 2023-02-16, 22 (3) [2024-02-06]. doi:10.1038/d41573-023-00030-y. (原始內容存檔於2024-04-09) (英語).
- ^ Zhao, Zixuan; Chen, Xinyi; Dowbaj, Anna M.; Sljukic, Aleksandra; Bratlie, Kaitlin; Lin, Luda; Fong, Eliza Li Shan; Balachander, Gowri Manohari; Chen, Zhaowei; Soragni, Alice; Huch, Meritxell. Organoids. Nature Reviews Methods Primers. 2022-12-01, 2 (1) [2024-02-05]. ISSN 2662-8449. doi:10.1038/s43586-022-00174-y. (原始內容存檔於2024-04-02) (英語).
- ^ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 Lancaster, Madeline A.; Knoblich, Juergen A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science. 2014-07-18, 345 (6194) [2024-02-06]. ISSN 0036-8075. doi:10.1126/science.1247125. (原始內容存檔於2024-04-03) (英語).
- ^ Wilson HV. A new method by which sponges may be artificially reared. Science. June 1907, 25 (649): 912–5. Bibcode:1907Sci....25..912W. PMID 17842577. doi:10.1126/science.25.649.912.
- ^ Holtfreter J. Experimental studies on the development of the pronephros.. Rev. Can. Biol. 1944, 3: 220–250.
- ^ Weiss P, Taylor AC. Reconstitution of complete organs from single-cell suspensions of chick embryos in advanced stages of differentiation. Proceedings of the National Academy of Sciences of the United States of America. September 1960, 46 (9): 1177–85. Bibcode:1960PNAS...46.1177W. PMC 223021
 . PMID 16590731. doi:10.1073/pnas.46.9.1177
. PMID 16590731. doi:10.1073/pnas.46.9.1177  .
.
- ^ Rheinwald, James G.; Green, Howard. Formation of a keratinizing epithelium in culture by a cloned cell line derived from a teratoma. Cell. November 1975, 6 (3): 317–330. ISSN 0092-8674. PMID 1052770. S2CID 28185779. doi:10.1016/0092-8674(75)90183-x.
- ^ 9.0 9.1 Yi, Sang Ah; Zhang, Yixiao; Rathnam, Christopher; Pongkulapa, Thanapat; Lee, Ki‐Bum. Bioengineering Approaches for the Advanced Organoid Research. Advanced Materials. 2021-11, 33 (45) [2024-02-06]. ISSN 0935-9648. PMC 8682947
 . PMID 34561899. doi:10.1002/adma.202007949. (原始內容存檔於2024-01-24) (英語).
. PMID 34561899. doi:10.1002/adma.202007949. (原始內容存檔於2024-01-24) (英語).
- ^ Li, M L; Aggeler, J; Farson, D A; Hatier, C; Hassell, J; Bissell, M J. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells.. Proceedings of the National Academy of Sciences. January 1987, 84 (1): 136–140. Bibcode:1987PNAS...84..136L. ISSN 0027-8424. PMC 304157
 . PMID 3467345. doi:10.1073/pnas.84.1.136
. PMID 3467345. doi:10.1073/pnas.84.1.136  .
.
- ^ Barcellos-Hoff, M. H.; Aggeler, J.; Ram, T. G.; Bissell, M. J. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989-02-01, 105 (2): 223–235. ISSN 0950-1991. PMC 2948482
 . PMID 2806122. doi:10.1242/dev.105.2.223.
. PMID 2806122. doi:10.1242/dev.105.2.223.
- ^ Hofer, Moritz; Lutolf, Matthias P. Engineering organoids. Nature Reviews Materials. 2021-05, 6 (5) [2024-02-05]. ISSN 2058-8437. doi:10.1038/s41578-021-00279-y. (原始內容存檔於2024-02-10) (英語).
- ^ Streuli, C H; Schmidhauser, C; Bailey, N; Yurchenco, P; Skubitz, A P; Roskelley, C; Bissell, M J. Laminin mediates tissue-specific gene expression in mammary epithelia.. The Journal of Cell Biology. 1995-05-01, 129 (3): 591–603. ISSN 0021-9525. PMC 2120432
 . PMID 7730398. doi:10.1083/jcb.129.3.591.
. PMID 7730398. doi:10.1083/jcb.129.3.591.
- ^ Nahmias Y, Schwartz RE, Hu WS, Verfaillie CM, Odde DJ. Endothelium-mediated hepatocyte recruitment in the establishment of liver-like tissue in vitro. Tissue Engineering. June 2006, 12 (6): 1627–38. PMID 16846358. doi:10.1089/ten.2006.12.1627.
- ^ Yong E. Lab-Grown Model Brains. The Scientist. August 28, 2013 [26 December 2013]. (原始內容存檔於2017-09-20).
- ^ 16.0 16.1 16.2 16.3 16.4 Sato, Toshiro; Vries, Robert G.; Snippert, Hugo J.; van de Wetering, Marc; Barker, Nick; Stange, Daniel E.; van Es, Johan H.; Abo, Arie; Kujala, Pekka; Peters, Peter J.; Clevers, Hans. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009-05, 459 (7244) [2024-02-06]. ISSN 1476-4687. doi:10.1038/nature07935. (原始內容存檔於2024-02-03) (英語).
- ^ 17.0 17.1 Unbekandt, Mathieu; Davies, Jamie A. Dissociation of embryonic kidneys followed by reaggregation allows the formation of renal tissues. Kidney International. 2010-03-01, 77 (5) [2024-02-06]. ISSN 0085-2538. doi:10.1038/ki.2009.482. (原始內容存檔於2024-02-06).
- ^ Peng H, Poovaiah N, Forrester M, Cochran E, Wang Q. Ex Vivo Culture of Primary Intestinal Stem Cells in Collagen Gels and Foams. ACS Biomaterials Science & Engineering. 2014 Dec 2;1(1):37–42. https://doi.org/10.1021/ab500041d. PMID: 33435081.
- ^ Peng H, Wang C, Xu X, Yu C, Wang Q. An intestinal Trojan horse for gene delivery. Nanoscale. 2015 Jan 6;7(10):4354–4360. https://doi.org/10.1039/C4NR06377E. PMID: 25619169.
- ^ Lawrence ML, Chang CH, Davies JA. Transport of organic anions and cations in murine embryonic kidney development and in serially-reaggregated engineered kidneys. Scientific Reports. March 2015, 5: 9092. Bibcode:2015NatSR...5E9092L. PMC 4357899
 . PMID 25766625. doi:10.1038/srep09092.
. PMID 25766625. doi:10.1038/srep09092.
- ^ Xinaris C, Benedetti V, Rizzo P, Abbate M, Corna D, Azzollini N, Conti S, Unbekandt M, Davies JA, Morigi M, Benigni A, Remuzzi G. In vivo maturation of functional renal organoids formed from embryonic cell suspensions. Journal of the American Society of Nephrology. November 2012, 23 (11): 1857–68. PMC 3482737
 . PMID 23085631. doi:10.1681/ASN.2012050505.
. PMID 23085631. doi:10.1681/ASN.2012050505.
- ^ Yui, S., Nakamura, T., Sato, T. et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nature Medicine 18, 618–623 (2012). https://doi.org/10.1038/nm.2695
- ^ 23.0 23.1 23.2 Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. September 2013, 501 (7467): 373–9. Bibcode:2013Natur.501..373L. PMC 3817409
 . PMID 23995685. doi:10.1038/nature12517.
. PMID 23995685. doi:10.1038/nature12517.
- ^ Huch, M., Dorrell, C., Boj, S. et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494, 247–250 (2013). https://doi.org/10.1038/nature11826
- ^ Shkumatov A, Baek K, Kong H. Matrix rigidity-modulated cardiovascular organoid formation from embryoid bodies. PLOS ONE. 2014, 9 (4): e94764. Bibcode:2014PLoSO...994764S. PMC 3986240
 . PMID 24732893. doi:10.1371/journal.pone.0094764
. PMID 24732893. doi:10.1371/journal.pone.0094764  .
.
- ^ Takebe T, Enomura M, Yoshizawa E, Kimura M, Koike H, Ueno Y, Matsuzaki T, Yamazaki T, Toyohara T, Osafune K, Nakauchi H, Yoshikawa HY, Taniguchi H. Vascularized and Complex Organ Buds from Diverse Tissues via Mesenchymal Cell-Driven Condensation. Cell Stem Cell. May 2015, 16 (5): 556–65. PMID 25891906. doi:10.1016/j.stem.2015.03.004
 .
.
- ^ Murry, Charles E.; Keller, Gordon. Differentiation of Embryonic Stem Cells to Clinically Relevant Populations: Lessons from Embryonic Development. Cell. February 2008, 132 (4): 661–680. ISSN 0092-8674. PMID 18295582. doi:10.1016/j.cell.2008.02.008
 .
.
- ^ Choudhury, Deepak; Ashok, Aswathi; Naing, May Win. Commercialization of Organoids. Trends in Molecular Medicine. March 2020, 26 (3): 245–249. ISSN 1471-4914. PMID 31982341. S2CID 210922708. doi:10.1016/j.molmed.2019.12.002.
- ^ Li ML, Aggeler J, Farson DA, Hatier C, Hassell J, Bissell MJ. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. January 1987, 84 (1): 136–40. Bibcode:1987PNAS...84..136L. PMC 304157
 . PMID 3467345. doi:10.1073/pnas.84.1.136
. PMID 3467345. doi:10.1073/pnas.84.1.136  .
.
- ^ 30.0 30.1 30.2 Pastuła A, Middelhoff M, Brandtner A, Tobiasch M, Höhl B, Nuber AH, Demir IE, Neupert S, Kollmann P, Mazzuoli-Weber G, Quante M. Three-Dimensional Gastrointestinal Organoid Culture in Combination with Nerves or Fibroblasts: A Method to Characterize the Gastrointestinal Stem Cell Niche. Stem Cells International. 2016, 2016: 3710836. PMC 4677245
 . PMID 26697073. doi:10.1155/2016/3710836
. PMID 26697073. doi:10.1155/2016/3710836  .
.
- ^ Takahashi, Kazutoshi; Yamanaka, Shinya. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. August 2006, 126 (4): 663–676 [2024-02-05]. ISSN 0092-8674. PMID 16904174. doi:10.1016/j.cell.2006.07.024. hdl:2433/159777
 . (原始內容存檔於2008-07-19).
. (原始內容存檔於2008-07-19).
- ^ Ran, F Ann; Hsu, Patrick D; Wright, Jason; Agarwala, Vineeta; Scott, David A; Zhang, Feng. Genome engineering using the CRISPR-Cas9 system. Nature Protocols. 2013-10-24, 8 (11): 2281–2308. ISSN 1754-2189. PMC 3969860
 . PMID 24157548. doi:10.1038/nprot.2013.143.
. PMID 24157548. doi:10.1038/nprot.2013.143.
- ^ Lavazza A, Massimini M. Cerebral organoids: ethical issues and consciousness assessment. Journal of Medical Ethics. September 2018, 44 (9): 606–610. PMID 29491041. doi:10.1136/medethics-2017-104555
 .
.
- ^ Prosser Scully, Ruby. Miniature brains grown in the lab have human-like neural activity. New Scientist (3237). 6 July 2019 [2024-02-05]. (原始內容存檔於2024-02-05).
- ^ Sample, Ian. Scientists 'may have crossed ethical line' in growing human brains. The Guardian. 21 October 2019: 15 [2024-02-05]. (原始內容存檔於2019-11-19).
- ^ Cai, Hongwei; Ao, Zheng; Tian, Chunhui; Wu, Zhuhao; Liu, Hongcheng; Tchieu, Jason; Gu, Mingxia; Mackie, Ken; Guo, Feng. Brain organoid reservoir computing for artificial intelligence. Nature Electronics. 2023-12, 6 (12) [2024-02-06]. ISSN 2520-1131. doi:10.1038/s41928-023-01069-w. (原始內容存檔於2024-02-23) (英語).
- ^ Smirnova, Lena; Caffo, Brian S.; Gracias, David H.; Huang, Qi; Morales Pantoja, Itzy E.; Tang, Bohao; Zack, Donald J.; Berlinicke, Cynthia A.; Boyd, J. Lomax; Harris, Timothy D.; Johnson, Erik C. Organoid intelligence (OI): the new frontier in biocomputing and intelligence-in-a-dish. Frontiers in Science. 2023, 1 [2024-02-06]. ISSN 2813-6330. doi:10.3389/fsci.2023.1017235. (原始內容存檔於2023-06-20).
- ^ Sato, Toshiro; Stange, Daniel E.; Ferrante, Marc; Vries, Robert G.J.; van Es, Johan H.; van den Brink, Stieneke; van Houdt, Winan J.; Pronk, Apollo; van Gorp, Joost; Siersema, Peter D.; Clevers, Hans. Long-term Expansion of Epithelial Organoids From Human Colon, Adenoma, Adenocarcinoma, and Barrett's Epithelium. Gastroenterology. November 2011, 141 (5): 1762–1772. ISSN 0016-5085. PMID 21889923. doi:10.1053/j.gastro.2011.07.050
 .
.
- ^ Jung, Peter; Sato, Toshiro; Merlos-Suárez, Anna; Barriga, Francisco M; Iglesias, Mar; Rossell, David; Auer, Herbert; Gallardo, Mercedes; Blasco, Maria A; Sancho, Elena; Clevers, Hans. Isolation and in vitro expansion of human colonic stem cells. Nature Medicine. October 2011, 17 (10): 1225–1227 [2024-02-05]. ISSN 1078-8956. PMID 21892181. S2CID 205388154. doi:10.1038/nm.2470. (原始內容存檔於2024-02-04) (英語).
- ^ Cai T, Qi Y, Jergens A, Wannemuehler M, Barrett TA, Wang Q. Effects of six common dietary nutrients on murine intestinal organoid growth. PLoS One. 2018 Feb 1;13(2):e0191517. https://doi.org/10.1371/journal.pone.0191517. PMID: 29389993; PMCID: PMC5794098.
- ^ Qi Y, Lohman J, Bratlie KM, Peroutka-Bigus N, Bellaire B, Wannemuehler M, Yoon KJ, Barrett TA, Wang Q. Vitamin C and B3 as new biomaterials to alter intestinal stem cells. Journal of Biomedical Materials Research Part A. 2019 Sep;107(9):1886–1897. https://doi.org/10.1002/jbm.a.36715. Epub 2019 May 23. PMID: 31071241; PMCID: PMC6626554.
- ^ Davoudi Z, Peroutka-Bigus N, Bellaire B, Wannemuehler M, Barrett TA, Narasimhan B, Wang Q. Intestinal organoids containing poly(lactic-co-glycolic acid) nanoparticles for the treatment of inflammatory bowel diseases. Journal of Biomedical Materials Research Part A. 2018 Apr;106(4):876–886. https://doi.org/10.1002/jbm.a.36305. Epub 2017 Dec 21. PMID: 29226615; PMCID: PMC5826879.
- ^ Davoudi Z, Peroutka-Bigus N, Bellaire B, Jergens A, Wannemuehler M, Wang Q. Gut Organoid as a New Platform to Study Alginate and Chitosan Mediated PLGA Nanoparticles for Drug Delivery. Marine Drugs. 2021 May 20;19(5):282. https://doi.org/10.3390/md19050282. PMID: 34065505; PMCID: PMC8161322.
- ^ Qi Y, Shi E, Peroutka-Bigus N, Bellaire B, Wannemuehler M, Jergens A, Barrett T, Wu Y, Wang Q. Ex Vivo Study of Telluride Nanowires in Minigut. Journal of Biomedical Nanotechnology. 2018 May 1;14(5):978–986. https://doi.org/10.1166/jbn.2018.2578. PMID: 29883567
- ^ Reding B, Carter P, Qi Y, Li Z, Wu Y, Wannemuehler M, Bratlie KM, Wang Q. Manipulate intestinal organoids with niobium carbide nanosheets. Journal of Biomedical Materials Research Part A. 2021 Apr;109(4):479–487. https://doi.org/10.1002/jbm.a.37032. Epub 2020 Jun 17. PMID: 32506610.
- ^ Zietek T, Rath E, Haller D, Daniel H. Intestinal organoids for assessing nutrient transport, sensing and incretin secretion. Scientific Reports. November 2015, 5 (1): 16831. Bibcode:2015NatSR...516831Z. PMC 4652176
 . PMID 26582215. doi:10.1038/srep16831
. PMID 26582215. doi:10.1038/srep16831  .
.
- ^ Zietek T, Giesbertz P, Ewers M, Reichart F, Weinmüller M, Demir IE, Haller D, Ceyhan GO, Kessler H, Rath E. Organoids to Study Intestinal Nutrient Transport, Drug Uptake and Metabolism – Update to the Human Model and Expansion of Applications. Frontiers in Bioengineering and Biotechnology. 2020, 8: 577656. PMC 7516017
 . PMID 33015026. doi:10.3389/fbioe.2020.577656
. PMID 33015026. doi:10.3389/fbioe.2020.577656  .
.
- ^ Rahmani, Sara; Breyner, Natalia M.; Su, Hsuan-Ming; Verdu, Elena F.; Didar, Tohid F. Intestinal organoids: A new paradigm for engineering intestinal epithelium in vitro. Biomaterials. 2019-02-01, 194: 195–214 [2024-02-05]. ISSN 0142-9612. PMID 30612006. S2CID 58603850. doi:10.1016/j.biomaterials.2018.12.006. (原始內容存檔於2024-04-21) (英語).
- ^ Sun L, Rollins D, Qi Y, Fredericks J, Mansell TJ, Jergens A, Phillips GJ, Wannemuehler M, Wang Q. TNFα regulates intestinal organoids from mice with both defined and conventional microbiota. International Journal of Biological Macromolecules. 2020 Dec 1;164:548–556. https://doi.org/10.1016/j.ijbiomac.2020.07.176. Epub 2020 Jul 18. PMID: 32693143; PMCID: PMC7657954.
- ^ Tong T, Qi Y, Rollins D, Bussiere LD, Dhar D, Miller CL, Yu C, Wang Q. Rational design of oral drugs targeting mucosa delivery with gut organoid platforms. Bioactive Materials. 2023; 30: 116–128. https://doi.org/10.1016/j.bioactmat.2023.07.014. PMID 37560199.
- ^ Davoudi Z, Atherly T, Borcherding DC, Jergens AE, Wannemuehler M, Barrett TA, Wang Q. Study Transportation of Drugs within Newly Established Murine Colon Organoid Systems. Advanced Biology. 2023; e2300103. https://doi.org/10.1002/adbi.202300103. PMID 37607116.
- ^ Lindemans C, Mertelsmann A, Dudakov JA, Velardi E, Hua G, O'Connor M, Kolesnick R, van den Brink MR, Hanash AM. IL-22 Administration Protects Intestinal Stem Cells from Gvhd. Biology of Blood and Marrow Transplantation. 2014, 20 (2): S53–S54. doi:10.1016/j.bbmt.2013.12.056
 .
.
- ^ Zhang, Yong-Guo; Wu, Shaoping; Xia, Yinglin; Sun, Jun. Salmonella -infected crypt-derived intestinal organoid culture system for host-bacterial interactions. Physiological Reports. September 2014, 2 (9): e12147. PMC 4270227
 . PMID 25214524. doi:10.14814/phy2.12147 (英語).
. PMID 25214524. doi:10.14814/phy2.12147 (英語).
- ^ Geiser, Petra; Di Martino, Maria Letizia; Samperio Ventayol, Pilar; Eriksson, Jens; Sima, Eduardo; Al-Saffar, Anas Kh.; Ahl, David; Phillipson, Mia; Webb, Dominic-Luc; Sundbom, Magnus; Hellström, Per M. Sperandio, Vanessa , 編. Salmonella enterica Serovar Typhimurium Exploits Cycling through Epithelial Cells To Colonize Human and Murine Enteroids. mBio. 2021-02-23, 12 (1). ISSN 2161-2129. PMC 7844539
 . PMID 33436434. doi:10.1128/mBio.02684-20 (英語).
. PMID 33436434. doi:10.1128/mBio.02684-20 (英語).
- ^ Dutta, Devanjali; Heo, Inha; O'Connor, Roberta. Studying Cryptosporidium Infection in 3D Tissue-derived Human Organoid Culture Systems by Microinjection. Journal of Visualized Experiments. 2019-09-14, (151): 59610. ISSN 1940-087X. PMID 31566619. S2CID 203377662. doi:10.3791/59610 (英語).
- ^ Co, Julia Y.; Margalef-Català, Mar; Li, Xingnan; Mah, Amanda T.; Kuo, Calvin J.; Monack, Denise M.; Amieva, Manuel R. Controlling Epithelial Polarity: A Human Enteroid Model for Host-Pathogen Interactions. Cell Reports. February 2019, 26 (9): 2509–2520.e4. PMC 6391775
 . PMID 30811997. doi:10.1016/j.celrep.2019.01.108 (英語).
. PMID 30811997. doi:10.1016/j.celrep.2019.01.108 (英語).
- ^ Tong T, Qi Y, Bussiere LD, Wannemuehler M, Miller CL, Wang Q, Yu C . Transport of artificial virus-like nanocarriers through intestinal monolayers via microfold cells. Nanoscale. 2020 Aug 14;12(30):16339-16347. https://doi.org/10.1039/D0NR03680C. Epub 2020 Jul 29. PMID 32725029.
- ^ 58.0 58.1 Noel, Gaelle; Baetz, Nicholas W.; Staab, Janet F.; Donowitz, Mark; Kovbasnjuk, Olga; Pasetti, Marcela F.; Zachos, Nicholas C. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Scientific Reports. 2017-05-31, 7 (1): 45270. Bibcode:2017NatSR...745270N. ISSN 2045-2322. PMC 5366908
 . PMID 28345602. doi:10.1038/srep45270 (英語).
. PMID 28345602. doi:10.1038/srep45270 (英語).
- ^ Bouchi R, Foo KS, Hua H, Tsuchiya K, Ohmura Y, Sandoval PR, Ratner LE, Egli D, Leibel RL, Accili D. FOXO1 inhibition yields functional insulin-producing cells in human gut organoid cultures. Nature Communications. June 2014, 5: 4242. Bibcode:2014NatCo...5.4242B. PMC 4083475
 . PMID 24979718. doi:10.1038/ncomms5242.
. PMID 24979718. doi:10.1038/ncomms5242.
- ^ Sontheimer-Phelps, Alexandra; Chou, David B.; Tovaglieri, Alessio; Ferrante, Thomas C.; Duckworth, Taylor; Fadel, Cicely; Frismantas, Viktoras; Sutherland, Arlene D.; Jalili-Firoozinezhad, Sasan; Kasendra, Magdalena; Stas, Eric. Human Colon-on-a-Chip Enables Continuous In Vitro Analysis of Colon Mucus Layer Accumulation and Physiology. Cellular and Molecular Gastroenterology and Hepatology. 2020, 9 (3): 507–526. PMC 7036549
 . PMID 31778828. doi:10.1016/j.jcmgh.2019.11.008 (英語).
. PMID 31778828. doi:10.1016/j.jcmgh.2019.11.008 (英語).
- ^ Grassart, Alexandre; Malardé, Valérie; Gobaa, Samy; Sartori-Rupp, Anna; Kerns, Jordan; Karalis, Katia; Marteyn, Benoit; Sansonetti, Philippe; Sauvonnet, Nathalie. Bioengineered Human Organ-on-Chip Reveals Intestinal Microenvironment and Mechanical Forces Impacting Shigella Infection. Cell Host & Microbe. September 2019, 26 (3): 435–444.e4. PMID 31492657. S2CID 201868491. doi:10.1016/j.chom.2019.08.007
 (英語).
(英語).
- ^ 62.0 62.1 McCracken KW, Catá EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE, Tsai YH, Mayhew CN, Spence JR, Zavros Y, Wells JM. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. December 2014, 516 (7531): 400–4. Bibcode:2014Natur.516..400M. PMC 4270898
 . PMID 25363776. doi:10.1038/nature13863.
. PMID 25363776. doi:10.1038/nature13863.
- ^ Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. January 2010, 6 (1): 25–36. PMID 20085740. doi:10.1016/j.stem.2009.11.013
 .
.
- ^ Li X, Nadauld L, Ootani A, Corney DC, Pai RK, Gevaert O, Cantrell MA, Rack PG, Neal JT, Chan CW, Yeung T, Gong X, Yuan J, Wilhelmy J, Robine S, Attardi LD, Plevritis SK, Hung KE, Chen CZ, Ji HP, Kuo CJ. Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nature Medicine. July 2014, 20 (7): 769–77. PMC 4087144
 . PMID 24859528. doi:10.1038/nm.3585.
. PMID 24859528. doi:10.1038/nm.3585.
- ^ 65.0 65.1 Nadauld LD, Garcia S, Natsoulis G, Bell JM, Miotke L, Hopmans ES, Xu H, Pai RK, Palm C, Regan JF, Chen H, Flaherty P, Ootani A, Zhang NR, Ford JM, Kuo CJ, Ji HP. Metastatic tumor evolution and organoid modeling implicate TGFBR2 as a cancer driver in diffuse gastric cancer. Genome Biology. August 2014, 15 (8): 428. PMC 4145231
 . PMID 25315765. doi:10.1186/s13059-014-0428-9
. PMID 25315765. doi:10.1186/s13059-014-0428-9  .
.
- ^ 66.0 66.1 Hisha H, Tanaka T, Kanno S, Tokuyama Y, Komai Y, Ohe S, Yanai H, Omachi T, Ueno H. Establishment of a novel lingual organoid culture system: generation of organoids having mature keratinized epithelium from adult epithelial stem cells. Scientific Reports. November 2013, 3: 3224. Bibcode:2013NatSR...3E3224H. PMC 3828633
 . PMID 24232854. doi:10.1038/srep03224.
. PMID 24232854. doi:10.1038/srep03224.
- ^ Aihara E, Mahe MM, Schumacher MA, Matthis AL, Feng R, Ren W, Noah TK, Matsu-ura T, Moore SR, Hong CI, Zavros Y, Herness S, Shroyer NF, Iwatsuki K, Jiang P, Helmrath MA, Montrose MH. Characterization of stem/progenitor cell cycle using murine circumvallate papilla taste bud organoid. Scientific Reports. November 2015, 5: 17185. Bibcode:2015NatSR...517185A. PMC 4665766
 . PMID 26597788. doi:10.1038/srep17185.
. PMID 26597788. doi:10.1038/srep17185.
- ^ Ren W, Lewandowski BC, Watson J, Aihara E, Iwatsuki K, Bachmanov AA, Margolskee RF, Jiang P. Single Lgr5- or Lgr6-expressing taste stem/progenitor cells generate taste bud cells ex vivo. Proceedings of the National Academy of Sciences of the United States of America. November 2014, 111 (46): 16401–6. Bibcode:2014PNAS..11116401R. PMC 4246268
 . PMID 25368147. doi:10.1073/pnas.1409064111
. PMID 25368147. doi:10.1073/pnas.1409064111  .
.
- ^ Martin A, Barbesino G, Davies TF. T-cell receptors and autoimmune thyroid disease—signposts for T-cell-antigen driven diseases. International Reviews of Immunology. 1999, 18 (1–2): 111–40. PMID 10614741. doi:10.3109/08830189909043021.
- ^ Bredenkamp N, Ulyanchenko S, O'Neill KE, Manley NR, Vaidya HJ, Blackburn CC. An organized and functional thymus generated from FOXN1-reprogrammed fibroblasts. Nature Cell Biology. September 2014, 16 (9): 902–8. PMC 4153409
 . PMID 25150981. doi:10.1038/ncb3023.
. PMID 25150981. doi:10.1038/ncb3023.
- ^ 71.0 71.1 71.2 Vianello F, Poznansky MC. Generation of a tissue-engineered thymic organoid. Methods in Molecular Biology 380. 2007: 163–70. ISBN 978-1-59745-395-0. PMID 17876092. doi:10.1385/1-59745-395-1:163 (不活躍 2024-01-24).
- ^ Sakib, Sadman; et al. Formation of organotypic testicular organoids in microwell culture. Biology of Reproduction. 1 June 2019, 100 (6): 1648–1660. PMC 7302515
 . PMID 30927418. doi:10.1093/biolre/ioz053.
. PMID 30927418. doi:10.1093/biolre/ioz053.
- ^ Drost, Jarno; Karthaus, Wouter R.; Gao, Dong; Driehuis, Else; Sawyers, Charles L.; Chen, Yu; Clevers, Hans. Organoid culture systems for prostate epithelial and cancer tissue. Nature Protocols. 21 January 2016, 11 (2): 347–358. ISSN 1750-2799. PMC 4793718
 . PMID 26797458. doi:10.1038/nprot.2016.006 (英語).
. PMID 26797458. doi:10.1038/nprot.2016.006 (英語).
- ^ Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, Ellis E, van Wenum M, Fuchs SA, de Ligt J, van de Wetering M, Sasaki N, Boers SJ, Kemperman H, de Jonge J, Ijzermans JN, Nieuwenhuis EE, Hoekstra R, Strom S, Vries RR, van der Laan LJ, Cuppen E, Clevers H. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. January 2015, 160 (1–2): 299–312. PMC 4313365
 . PMID 25533785. doi:10.1016/j.cell.2014.11.050.
. PMID 25533785. doi:10.1016/j.cell.2014.11.050.
- ^ Li P, Li Y, Wang Y, Liu J, Lavrijsen M, Li Y, Zhang R, Verstegen MMA, Wang Y, Li TC, Ma Z, Kainov DE, Bruno MJ, de Man RA, van der Laan LJW, Peppelenbosch MP, Pan Q. Recapitulating hepatitis E virus-host interactions and facilitating antiviral drug discovery in human liver-derived organoids. Science Advances. 2022, 8 (3): 103–111. Bibcode:2022SciA....8.5908L. PMID 5044825. S2CID 246069868. doi:10.1126/sciadv.abj5908. hdl:11250/3047921
 .
.
- ^ Huch M, Bonfanti P, Boj SF, Sato T, Loomans CJ, van de Wetering M, Sojoodi M, Li VS, Schuijers J, Gracanin A, Ringnalda F, Begthel H, Hamer K, Mulder J, van Es JH, de Koning E, Vries RG, Heimberg H, Clevers H. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. The EMBO Journal. October 2013, 32 (20): 2708–21. PMC 3801438
 . PMID 24045232. doi:10.1038/emboj.2013.204.
. PMID 24045232. doi:10.1038/emboj.2013.204.
- ^ Hou S, Tiriac H, Sridharan BP, Scampavia L, Madoux F, Seldin J; et al. Advanced Development of Primary Pancreatic Organoid Tumor Models for High-Throughput Phenotypic Drug Screening.. SLAS Discov. 2018, 23 (6): 574–584. PMC 6013403
 . PMID 29673279. doi:10.1177/2472555218766842.
. PMID 29673279. doi:10.1177/2472555218766842.
- ^ Wolff RA, Wang-Gillam A, Alvarez H, Tiriac H, Engle D, Hou S; et al. Dynamic changes during the treatment of pancreatic cancer.. Oncotarget. 2018, 9 (19): 14764–14790. PMC 5871077
 . PMID 29599906. doi:10.18632/oncotarget.24483.
. PMID 29599906. doi:10.18632/oncotarget.24483.
- ^ Below, Christopher R.; Kelly, Joanna; Brown, Alexander; Humphries, Jonathan D.; Hutton, Colin; Xu, Jingshu; Lee, Brian Y.; Cintas, Celia; Zhang, Xiaohong; Hernandez-Gordillo, Victor; Stockdale, Linda. A microenvironment-inspired synthetic three-dimensional model for pancreatic ductal adenocarcinoma organoids. Nature Materials. 2021-09-13, 21 (1): 110–119. ISSN 1476-4660. PMC 7612137
 . PMID 34518665. doi:10.1038/s41563-021-01085-1 (英語).
. PMID 34518665. doi:10.1038/s41563-021-01085-1 (英語).
- ^ Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. October 2007, 449 (7165): 1003–7. Bibcode:2007Natur.449.1003B. PMID 17934449. S2CID 4349637. doi:10.1038/nature06196.
- ^ Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, Wagers AJ, Tseng YH, Ryeom S, Kim CF. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. January 2014, 156 (3): 440–55. PMC 3951122
 . PMID 24485453. doi:10.1016/j.cell.2013.12.039.
. PMID 24485453. doi:10.1016/j.cell.2013.12.039.
- ^ Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, Little MH. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. October 2015, 526 (7574): 564–8. Bibcode:2015Natur.526..564T. PMID 26444236. S2CID 4443766. doi:10.1038/nature15695.
- ^ 83.0 83.1 83.2 Freedman, Benjamin S.; Brooks, Craig R.; Lam, Albert Q.; Fu, Hongxia; Morizane, Ryuji; Agrawal, Vishesh; Saad, Abdelaziz F.; Li, Michelle K.; Hughes, Michael R.; Werff, Ryan Vander; Peters, Derek T. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nature Communications. 2015-10-23, 6 (1) [2024-02-06]. ISSN 2041-1723. doi:10.1038/ncomms9715. (原始內容存檔於2024-02-06) (英語).
- ^ Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nature Biotechnology. November 2015, 33 (11): 1193–200. PMC 4747858
 . PMID 26458176. doi:10.1038/nbt.3392.
. PMID 26458176. doi:10.1038/nbt.3392.
- ^ van den Brink SC, Baillie-Johnson P, Balayo T, Hadjantonakis AK, Nowotschin S, Turner DA, Martinez Arias A. Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. Development. November 2014, 141 (22): 4231–42. PMC 4302915
 . PMID 25371360. doi:10.1242/dev.113001.
. PMID 25371360. doi:10.1242/dev.113001.
- ^ Turner DA, Baillie-Johnson P, Martinez Arias A. Organoids and the genetically encoded self-assembly of embryonic stem cells. BioEssays. February 2016, 38 (2): 181–91. PMC 4737349
 . PMID 26666846. doi:10.1002/bies.201500111.
. PMID 26666846. doi:10.1002/bies.201500111.
- ^ Turner DA, Girgin M, Alonso-Crisostomo L, Trivedi V, Baillie-Johnson P, Glodowski CR, Hayward PC, Collignon J, Gustavsen C, Serup P, Steventon B, P Lutolf M, Arias AM. Anteroposterior polarity and elongation in the absence of extra-embryonic tissues and of spatially localised signalling in gastruloids: mammalian embryonic organoids. Development. November 2017, 144 (21): 3894–3906. PMC 5702072
 . PMID 28951435. doi:10.1242/dev.150391.
. PMID 28951435. doi:10.1242/dev.150391.
- ^ 88.0 88.1 Beccari, Leonardo; Moris, Naomi; Girgin, Mehmet; Turner, David A.; Baillie-Johnson, Peter; Cossy, Anne-Catherine; Lutolf, Matthias P.; Duboule, Denis; Arias, Alfonso Martinez. Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature. 2018-10, 562 (7726) [2024-02-05]. ISSN 1476-4687. doi:10.1038/s41586-018-0578-0. (原始內容存檔於2024-01-17) (英語).
- ^ Blastoid: The backstory of the formation of blastocyst-like structure solely from stem cells. 2018-06-27 [2024-02-05]. (原始內容存檔於2019-10-25).
- ^ Nicolas Rivron Lab | Blastoid | Netherlands. [2024-02-05]. (原始內容存檔於2019-10-25).
- ^ Rivron NC, Frias-Aldeguer J, Vrij EJ, Boisset JC, Korving J, Vivié J, Truckenmüller RK, van Oudenaarden A, van Blitterswijk CA, Geijsen N. Blastocyst-like structures generated solely from stem cells (PDF). Nature. May 2018, 557 (7703): 106–111 [2024-02-05]. Bibcode:2018Natur.557..106R. PMID 29720634. S2CID 13749109. doi:10.1038/s41586-018-0051-0. (原始內容存檔 (PDF)於2024-02-05).
- ^ Rawlings TM, Makwana K, Tryfonos M, Lucas ES. Organoids to model the endometrium: implantation and beyond. Reprod Fertil. July 2021, 2 (3): R85–R101. PMC 8801025
 . PMID 35118399. doi:10.1530/RAF-21-0023.
. PMID 35118399. doi:10.1530/RAF-21-0023.
- ^ Lee EJ, Kim DE, Azeloglu EU, Costa KD. Engineered cardiac organoid chambers: toward a functional biological model ventricle. Tissue Engineering. Part A. February 2008, 14 (2): 215–25. PMID 18333774. doi:10.1089/tea.2007.0351.
- ^ Molteni, Megan. These Beating Mini-Hearts Could Save Big Bucks—And Maybe Lives. WIRED. 2018-06-27 [2018-06-30]. (原始內容存檔於2023-06-11).
- ^ Wiley LA, Burnight ER, DeLuca AP, Anfinson KR, Cranston CM, Kaalberg EE, Penticoff JA, Affatigato LM, Mullins RF, Stone EM, Tucker BA. cGMP production of patient-specific iPSCs and photoreceptor precursor cells to treat retinal degenerative blindness. Scientific Reports. July 2016, 6: 30742. Bibcode:2016NatSR...630742W. PMC 4965859
 . PMID 27471043. doi:10.1038/srep30742.
. PMID 27471043. doi:10.1038/srep30742.
- ^ Zilova, Lucie; Weinhardt, Venera; Tavhelidse, Tinatini; Schlagheck, Christina; Thumberger, Thomas; Wittbrodt, Joachim. Martínez Arias, Alfonso; Stainier, Didier YR; Martínez Arias, Alfonso , 編. Fish primary embryonic pluripotent cells assemble into retinal tissue mirroring in vivo early eye development. eLife. 2021-07-12, 10: e66998. ISSN 2050-084X. PMC 8275126
 . PMID 34252023. doi:10.7554/eLife.66998
. PMID 34252023. doi:10.7554/eLife.66998  .
.
- ^ Sachs, Norman; de Ligt, Joep; Kopper, Oded; Gogola, Ewa; Bounova, Gergana; Weeber, Fleur; Balgobind, Anjali Vanita; Wind, Karin; Gracanin, Ana; Begthel, Harry; Korving, Jeroen. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell. 2018, 172 (1–2): 373–386.e10. ISSN 0092-8674. PMID 29224780. doi:10.1016/j.cell.2017.11.010
 .
.
- ^ van de Wetering, Marc; Francies, Hayley; Francis, Joshua; Bounova, Gergana; Iorio, Francesco; Pronk, Apollo; van Houdt, Winan; van Gorp, Joost; Taylor-Weiner, Amaro; Kester, Lennart; McLaren-Douglas, Anne. Prospective Derivation of a Living Organoid Biobank of Colorectal Cancer Patients. Cell. 2015, 161 (4): 933–945. ISSN 0092-8674. PMC 6428276
 . PMID 25957691. doi:10.1016/j.cell.2015.03.053.
. PMID 25957691. doi:10.1016/j.cell.2015.03.053.
- ^ Quereda V, Hou S, Madoux F, Scampavia L, Spicer TP, Duckett D. A Cytotoxic Three-Dimensional-Spheroid, High-Throughput Assay Using Patient-Derived Glioma Stem Cells.. SLAS Discov. 2018, 23 (8): 842–849. PMC 6102052
 . PMID 29750582. doi:10.1177/2472555218775055.
. PMID 29750582. doi:10.1177/2472555218775055.
- ^ Dayton, Talya L.; Alcala, Nicolas; Moonen, Laura; den Hartigh, Lisanne; Geurts, Veerle; Mangiante, Lise; Lap, Lisa; Dost, Antonella F.M.; Beumer, Joep; Levy, Sonja; van Leeuwaarde, Rachel S. Druggable growth dependencies and tumor evolution analysis in patient-derived organoids of neuroendocrine neoplasms from multiple body sites. Cancer Cell. 2023, 41 (12): 2083–2099. ISSN 1535-6108. PMID 38086335. doi:10.1016/j.ccell.2023.11.007
 .
.
- ^ James, Owen G.; Selvaraj, Bhuvaneish T.; Magnani, Dario; Burr, Karen; Connick, Peter; Barton, Samantha K.; Vasistha, Navneet A.; Hampton, David W.; Story, David; Smigiel, Robert; Ploski, Rafal. iPSC-derived myelinoids to study myelin biology of humans. Developmental Cell. 2021-05-03, 56 (9) [2024-02-05]. ISSN 1534-5807. doi:10.1016/j.devcel.2021.04.006. (原始內容存檔於2024-04-18).
- ^ Zidarič, Tanja; Gradišnik, Lidija; Velnar, Tomaž. Astrocytes and human artificial blood-brain barrier models. Bosnian Journal of Basic Medical Sciences. 2022-04-01, 22 (5): 651–672 [2024-02-05]. ISSN 1840-4812. PMC 9519155
 . PMID 35366791. doi:10.17305/bjbms.2021.6943. (原始內容存檔於2024-02-05) (英語).
. PMID 35366791. doi:10.17305/bjbms.2021.6943. (原始內容存檔於2024-02-05) (英語).
- ^ Zidarič, Tanja; Gradišnik, Lidija; Velnar, Tomaž. Astrocytes and human artificial blood-brain barrier models. Bosnian Journal of Basic Medical Sciences. 2022-04-01, 22 (5): 651–672 [2024-02-05]. ISSN 1840-4812. PMC 9519155
 . PMID 35366791. doi:10.17305/bjbms.2021.6943. (原始內容存檔於2024-02-05) (英語).
. PMID 35366791. doi:10.17305/bjbms.2021.6943. (原始內容存檔於2024-02-05) (英語).
- ^ Zietek T, Rath E, Haller D, Daniel H. Intestinal organoids for assessing nutrient transport, sensing and incretin secretion. Scientific Reports. November 2015, 5: 16831. Bibcode:2015NatSR...516831Z. PMC 4652176
 . PMID 26582215. doi:10.1038/srep16831.
. PMID 26582215. doi:10.1038/srep16831.
- ^ 105.0 105.1 105.2 Cruz NM, Song X, Czerniecki SM, Gulieva RE, Churchill AJ, Kim YK, Winston K, Tran LM, Diaz MA, Fu H, Finn LS, Pei Y, Himmelfarb J, Freedman BS. Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease. Nature Materials. November 2017, 16 (11): 1112–1119. Bibcode:2017NatMa..16.1112C. PMC 5936694
 . PMID 28967916. doi:10.1038/nmat4994.
. PMID 28967916. doi:10.1038/nmat4994.
- ^ 106.0 106.1 106.2 Kim YK, Refaeli I, Brooks CR, Jing P, Gulieva RE, Hughes MR, Cruz NM, Liu Y, Churchill AJ, Wang Y, Fu H, Pippin JW, Lin LY, Shankland SJ, Vogl AW, McNagny KM, Freedman BS. Gene-Edited Human Kidney Organoids Reveal Mechanisms of Disease in Podocyte Development. Stem Cells. December 2017, 35 (12): 2366–2378. PMC 5742857
 . PMID 28905451. doi:10.1002/stem.2707.
. PMID 28905451. doi:10.1002/stem.2707.
- ^ Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors (PDF). Cell. November 2007, 131 (5): 861–72 [2024-02-05]. PMID 18035408. S2CID 8531539. doi:10.1016/j.cell.2007.11.019. hdl:2433/49782
 . (原始內容存檔 (PDF)於2024-02-11).
. (原始內容存檔 (PDF)於2024-02-11).
- ^ 108.0 108.1 108.2 Hsieh WC, Ramadesikan S, Fekete D, Aguilar RC. Kidney-differentiated cells derived from Lowe Syndrome patient's iPSCs show ciliogenesis defects and Six2 retention at the Golgi complex. PLOS ONE. 2018-02-14, 13 (2): e0192635. Bibcode:2018PLoSO..1392635H. PMC 5812626
 . PMID 29444177. doi:10.1371/journal.pone.0192635
. PMID 29444177. doi:10.1371/journal.pone.0192635  .
.
- ^ Howden SE, Thomson JA, Little MH. Simultaneous reprogramming and gene editing of human fibroblasts. Nature Protocols. May 2018, 13 (5): 875–898. PMC 5997775
 . PMID 29622803. doi:10.1038/nprot.2018.007.
. PMID 29622803. doi:10.1038/nprot.2018.007.
- ^ 110.0 110.1 110.2 110.3 Forbes TA, Howden SE, Lawlor K, Phipson B, Maksimovic J, Hale L, Wilson S, Quinlan C, Ho G, Holman K, Bennetts B, Crawford J, Trnka P, Oshlack A, Patel C, Mallett A, Simons C, Little MH. Patient-iPSC-Derived Kidney Organoids Show Functional Validation of a Ciliopathic Renal Phenotype and Reveal Underlying Pathogenetic Mechanisms. American Journal of Human Genetics. May 2018, 102 (5): 816–831. PMC 5986969
 . PMID 29706353. doi:10.1016/j.ajhg.2018.03.014.
. PMID 29706353. doi:10.1016/j.ajhg.2018.03.014.
- ^ 111.0 111.1 Tanigawa S, Islam M, Sharmin S, Naganuma H, Yoshimura Y, Haque F, Era T, Nakazato H, Nakanishi K, Sakuma T, Yamamoto T, Kurihara H, Taguchi A, Nishinakamura R. Organoids from Nephrotic Disease-Derived iPSCs Identify Impaired NEPHRIN Localization and Slit Diaphragm Formation in Kidney Podocytes. Stem Cell Reports. September 2018, 11 (3): 727–740. PMC 6135868
 . PMID 30174315. doi:10.1016/j.stemcr.2018.08.003.
. PMID 30174315. doi:10.1016/j.stemcr.2018.08.003.
- ^ Engle SJ, Blaha L, Kleiman RJ. Best Practices for Translational Disease Modeling Using Human iPSC-Derived Neurons. Neuron. November 2018, 100 (4): 783–797. PMID 30465765. doi:10.1016/j.neuron.2018.10.033
 .
.
- ^ Metabolites. www.mdpi.com. [2022-10-16]. (原始內容存檔於2022-10-18) (英語).
- ^ 114.0 114.1 Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NW, Bijvelds MJ, Scholte BJ, Nieuwenhuis EE, van den Brink S, Clevers H, van der Ent CK, Middendorp S, Beekman JM. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nature Medicine. July 2013, 19 (7): 939–45. PMID 23727931. S2CID 5369669. doi:10.1038/nm.3201.
- ^ 115.0 115.1 Dekkers JF, Berkers G, Kruisselbrink E, Vonk A, de Jonge HR, Janssens HM, Bronsveld I, van de Graaf EA, Nieuwenhuis EE, Houwen RH, Vleggaar FP, Escher JC, de Rijke YB, Majoor CJ, Heijerman HG, de Winter-de Groot KM, Clevers H, van der Ent CK, Beekman JM. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Science Translational Medicine. June 2016, 8 (344): 344ra84. PMID 27334259. S2CID 19462535. doi:10.1126/scitranslmed.aad8278.
- ^ Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK, Nieuwenhuis EE, Beekman JM, Clevers H. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. December 2013, 13 (6): 653–8. PMID 24315439. doi:10.1016/j.stem.2013.11.002
 .
.
- ^ World's first mini organ transportation to a patient with ulcerative colitis. Medical Xpress. 2022-08-22 [2024-02-06]. (原始內容存檔於2023-10-05) (英語).
- ^ Watanabe, Satoshi; Kobayashi, Sakurako; Ogasawara, Nobuhiko; Okamoto, Ryuichi; Nakamura, Tetsuya; Watanabe, Mamoru; Jensen, Kim B.; Yui, Shiro. Transplantation of intestinal organoids into a mouse model of colitis
 . Nature Protocols. March 2022, 17 (3): 649–671 [2024-02-05]. ISSN 1750-2799. PMID 35110738. S2CID 246488596. doi:10.1038/s41596-021-00658-3. (原始內容存檔於2023-10-06) (英語).
. Nature Protocols. March 2022, 17 (3): 649–671 [2024-02-05]. ISSN 1750-2799. PMID 35110738. S2CID 246488596. doi:10.1038/s41596-021-00658-3. (原始內容存檔於2023-10-06) (英語).
- ^ 119.0 119.1 119.2 119.3 119.4 Ader M, Tanaka EM. Modeling human development in 3D culture. Current Opinion in Cell Biology. December 2014, 31: 23–8. PMID 25033469. doi:10.1016/j.ceb.2014.06.013.
- ^ Martinez-Morales JR, Cavodeassi F, Bovolenta P. Coordinated Morphogenetic Mechanisms Shape the Vertebrate Eye. Frontiers in Neuroscience. 2017, 11: 721. PMC 5742352
 . PMID 29326547. doi:10.3389/fnins.2017.00721
. PMID 29326547. doi:10.3389/fnins.2017.00721  .
.
- ^ Gordon, Aaron; Yoon, Se-Jin; Tran, Stephen S.; Makinson, Christopher D.; Park, Jin Young; Andersen, Jimena; Valencia, Alfredo M.; Horvath, Steve; Xiao, Xinshu; Huguenard, John R.; Pașca, Sergiu P. Long-term maturation of human cortical organoids matches key early postnatal transitions. Nature Neuroscience. 2021-02-22, 24 (3): 331–342. ISSN 1546-1726. PMC 8109149
 . PMID 33619405. doi:10.1038/s41593-021-00802-y (英語).
. PMID 33619405. doi:10.1038/s41593-021-00802-y (英語).
