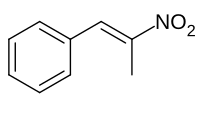
(2-硝基丙烯基)苯
外觀
| (2-硝基丙烯基)苯 | |
|---|---|
| |

| |
| IUPAC名 (2-Nitroprop-1-en-1-yl)benzene | |
| 別名 | P2NP 1-苯基-2-硝基丙烯 β-甲基-β-硝基苯乙烯 β-硝基丙烯基苯 |
| 識別 | |
| CAS號 | 705-60-2 |
| PubChem | 1549520 |
| ChemSpider | 1266396 |
| SMILES |
|
| InChI |
|
| InChIKey | WGSVFWFSJDAYBM-BQYQJAHWBX |
| 性質 | |
| 化學式 | C9H9NO2 |
| 摩爾質量 | 163.17 g mol−1 g·mol⁻¹ |
| 外觀 | 固體 |
| 熔點 | 65—66 °C(338—339 K)[1] |
| 危險性 | |
GHS危險性符號
| |
| GHS提示詞 | 警告 |
| H-術語 | H302, H315, H319, H335 |
| P-術語 | P261, P264, P280, P301+312, P312, P302+352, P304+340, P305+351+338, P330, P332+313, P337+313 |
| 若非註明,所有數據均出自標準狀態(25 ℃,100 kPa)下。 | |
(2-硝基丙烯基)苯是一種有機化合物,化學式為C9H9NO2。它可由苯甲醛和硝基乙烷在催化下反應製得。[2]在不同的反應條件下,它還原可以得到(2-硝基丙基)苯[3]、苯基丙酮[4]、苯基丙酮肟[5]或苯丙胺[6]。
參考文獻
[編輯]- ^ Gordon A. Alles. dl-BETA-PHENYLISOPROPYLAMINES. Journal of the American Chemical Society. 1932-01, 54 (1): 271–274 [2022-03-15]. ISSN 0002-7863. doi:10.1021/ja01340a040. (原始內容存檔於2022-03-15) (英語).
- ^ Kantam, M. Lakshmi; Sreekanth, P. One-pot synthesis of conjugated nitroalkenes by diamino-functionalized mesoporous material. Catalysis Letters. 1999. 57 (4): 227-231. ISSN 1011-372X.
- ^ Dong Xu, Yang Chen, Changmeng Liu, Jiaxi Xu, Zhanhui Yang. Iridium-catalyzed highly chemoselective and efficient reduction of nitroalkenes to nitroalkanes in water. Green Chemistry. 2021, 23 (16): 6050–6058 [2022-03-15]. ISSN 1463-9262. doi:10.1039/D1GC01907D (英語).
- ^ J.M. Aizpurua, M. Oiarbide, C. Palomo. Reduction of α,β-unsaturated nitrocompounds with tributyltin hydride.. Tetrahedron Letters. 1987-01, 28 (44): 5365–5366 [2022-03-15]. doi:10.1016/S0040-4039(00)96731-5. (原始內容存檔於2019-07-19) (英語).
- ^ Yujing Ren, Haisheng Wei, Guangzhao Yin, Leilei Zhang, Aiqin Wang, Tao Zhang. Oxygen surface groups of activated carbon steer the chemoselective hydrogenation of substituted nitroarenes over nickel nanoparticles. Chemical Communications. 2017, 53 (12): 1969–1972 [2022-03-15]. ISSN 1359-7345. doi:10.1039/C6CC08505A (英語).
- ^ Andersson, Sven Goran Berttil; Holmberg, Hans Viktor; Nilsson, Lars Anders Ragnar. Arylethylamines. 1984 GB 2122617 A.
