使用者:CantFinishExamPaper/苯胺紫
對不起哥們真不懂化學求化學大佬來改

苯胺紫,又稱珀金紫,是最早的合成染料之一。[1][2]1856年,威廉·珀金在嘗試合成用於治療瘧疾的植物化學物質奎寧時偶然發現了苯胺紫。[3]它也是最早批量生產的化學染料之一。[4][5]
化學
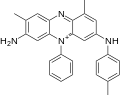
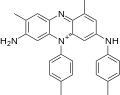
[編輯]苯胺紫是四種關聯芳香族化合物的混合物,其甲基的數量和位置不同。一種有機合成苯胺紫的例子是將苯胺、對甲苯胺和鄰甲苯胺以約1:1:2的比例溶解在硫酸和水中,然後添加重鉻酸鉀。[6]
苯胺紫A(C
26H
23N+
4X−
)分子包含1個苯胺、1個對甲苯胺和1個鄰甲苯胺。苯胺紫B(C
27H
25N+
4X−
)分子包含1個苯胺、1個對甲苯胺和2個鄰甲苯胺。1879年,珀金通過對甲苯胺的氧化/還原損失發現苯胺紫B與藏紅相關。[7]實際上,藏紅是一種2,8-二甲基非那嗪鹽,而珀金生產的番茄紅被認為是[8]1,8-(或2,9-)二甲基異構體。
The molecular structure of mauveine proved difficult to determine, finally being identified in 1994.[9] In 2007, two more were isolated and identified: mauveine B2, an isomer of mauveine B with methyl on different aryl group, and mauveine C, which has one more p-methyl group than mauveine A.[10]
-
Skeletal formula of mauveine A
-
Skeletal formula of mauveine B
-
Skeletal formula of mauveine B2
-
Skeletal formula of mauveine C
In 2008, additional mauveines and pseudomauveines were discovered, bringing the total number of these compounds up to 12.[11] In 2015 a crystal structure was reported for the first time.[12]
歷史
[編輯]
Mauveine #8D029B
In 1856, William Henry Perkin, then age 18, was given a challenge by his professor, August Wilhelm von Hofmann, to synthesize quinine. In one attempt, Perkin oxidized aniline using potassium dichromate, whose toluidine impurities reacted with the aniline and yielded a black solid, suggesting a "failed" organic synthesis. Cleaning the flask with alcohol, Perkin noticed purple portions of the solution.
Suitable as a dye of silk and other textiles, it was patented by Perkin, who the next year opened a dyeworks mass-producing it at Greenford on the banks of the Grand Union Canal in Middlesex.[13] It was originally called aniline purple. In 1859, it was named mauve in England via the French name for the mallow flower, and chemists later called it mauveine.[14] Between 1859 and 1861, mauve became a fashion must have. The weekly journal All the Year Round described women wearing the colour as "all flying countryward, like so many migrating birds of purple paradise".[15] Punch magazine published cartoons poking fun at the huge popularity of the colour 「The Mauve Measles are spreading to so serious an extent that it is high time to consider by what means [they] may be checked.」[16][17][18]
By 1870, demand succumbed to newer synthetic colors in the synthetic dye industry launched by mauveine.
In the early 20th century, the U.S. National Association of Confectioners permitted mauveine as a food coloring with a variety of equivalent names: rosolan, violet paste, chrome violet, anilin violet, anilin purple, Perkin's violet, indisin, phenamin, purpurin and lydin.[19]
Laborers in the aniline dye industry were later found to be at increased risk of bladder cancer, specifically transitional cell carcinoma, yet by the 1950s, the synthetic dye industry had helped transform medicine, including cancer treatment.[20][21][22]
引用
[編輯]- ^ Hubner. History – 150 Years of mauveine. Chemie in unserer Zeit. 2006, 40 (4): 274–275. doi:10.1002/ciuz.200690054.
- ^ Anthony S. Travis. Perkin's Mauve: Ancestor of the Organic Chemical Industry. Technology and Culture. 1990, 31 (1): 51–82. JSTOR 3105760. doi:10.2307/3105760.
- ^ St. Clair, Kassia. The Secret Lives of Colour. London: John Murray. 2016: 21. ISBN 9781473630819. OCLC 936144129.
- ^ Hicks, Jan. William Henry Perkin and the world's first synthetic dye. Science and Industry Museum blog. 2017-08-25 [2019-10-07] (英國英語).
- ^ The color purple: How an accidental discovery changed fashion forever. CNN. 12 March 2018.
- ^ A Microscale Synthesis of Mauve Scaccia, Rhonda L.; Coughlin, David; Ball, David W. J. Chem. Educ. 1998 75 769 Abstract
- ^ Perkin, W. H. On mauveine and allied colouring matters. J. Chem. Soc. Trans. January 1879, 1879: 717–732. doi:10.1039/CT8793500717.
- ^ Website source: ch.ic.ac.uk Link
- ^ Meth-Cohn, O.; Smith, M. What did W. H. Perkin actually make when he oxidised aniline to obtain mauveine?. Journal of the Chemical Society, Perkin Transactions 1. 1994, 1994: 5–7. doi:10.1039/P19940000005.
- ^ Seixas De Melo, J.; Takato, S.; Sousa, M.; Melo, M. J.; Parola, A. J. Revisiting Perkin's dye(s): The spectroscopy and photophysics of two new mauveine compounds (B2 and C). Chemical Communications. 2007, (25): 2624–6. PMID 17579759. doi:10.1039/b618926a.
- ^ Sousa, Micaela M.; Melo, Maria J.; Parola, A. Jorge; Morris, Peter J. T.; Rzepa, Henry S.; De Melo, J. Sérgio Seixas. A Study in Mauve: Unveiling Perkin's Dye in Historic Samples. Chemistry - A European Journal. 2008, 14 (28): 8507–8513. PMID 18671308. doi:10.1002/chem.200800718. hdl:10316/8229
 .
.
- ^ Plater, M. John; Harrison, William T. A.; Rzepa, Henry S. Syntheses and Structures of Pseudo-Mauveine Picrate and 3-Phenylamino-5-(2-Methylphenyl)-7-Amino-8-Methylphenazinium Picrate Ethanol Mono-Solvate: The First Crystal Structures of a Mauveine Chromophore and a Synthetic Derivative. Journal of Chemical Research. 2015, 39 (12): 711–718. doi:10.3184/174751915X14474318419130
 .
.
- ^ Google Earth location: Download
- ^ Matthew, H.C.G.; Howard Harrison, Brian. Oxford Dictionary of National Biography: In Association with the British Academy
 . Oxford University Press. 2004. ISBN 0-19-861393-8.
. Oxford University Press. 2004. ISBN 0-19-861393-8. perkins tyrian.purple.
- ^ Garfield, Simon. Simon Garfield on mauve. The Guardian. 2000-09-21 [2020-05-27]. ISSN 0261-3077 (英國英語).
- ^ Blakemore, Erin. How Malaria Gave Us Mauve. Smithsonian Magazine. [2020-05-27] (英語).
- ^ Jackson, Shelley. Colors / Mauve | Shelley Jackson. cabinetmagazine.org. [2020-05-27] (英語).
- ^ Day dress | V&A Search the Collections. V and A Collections. 2020-05-27 [2020-05-27] (英語).
- ^ Leffmann, Henry; William Beam. Select Methods in Food Analysis. Philadelphia: P. Blakiston's Son & Co. 1901: 77.
perkins tyrian.purple.
- ^ Cartwright, R.A. Historical and modern epidemiological studies on populations exposed to N-substituted aryl compounds. Environmental Health Perspectives. 1983, 49: 13–19. PMC 1569142
 . PMID 6339220. doi:10.1289/ehp.834913.
. PMID 6339220. doi:10.1289/ehp.834913.
- ^ John E Lesch, The First Miracle Drugs: How the Sulfa Drugs Transformed Medicine (New York: Oxford University Press, 2007), pp 202–3
- ^ D J Th Wagener, The History of Oncology (Houten: Springer, 2009), pp 150–1.
延伸閱讀
[編輯]- Simon Garfield. Mauve: How One Man Invented a Color That Changed the World
 . W. W. Norton & Company. 2002. ISBN 978-0393323139.
. W. W. Norton & Company. 2002. ISBN 978-0393323139.
外部連結
[編輯]- Perkin anniversary website 網際網路檔案館的存檔,存檔日期2006-11-11.
- Rotatable 3D models of mauveine are available using Jmol