賽他發定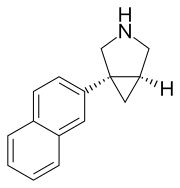 |
|
| 法律規範 |
- 美:Investigational New Drug
|
|---|
|
(1R,5S)-1-naphthalen-2-yl-3-azabicyclo[3.1.0]hexane
|
| CAS號 | 924012-43-1 |
|---|
| PubChem CID | |
|---|
| ChemSpider | |
|---|
| UNII | |
|---|
| KEGG | |
|---|
| CompTox Dashboard (EPA) | |
|---|
|
| 化學式 | C15H15N |
|---|
| 摩爾質量 | 209.29 g·mol−1 |
|---|
| 3D模型(JSmol) | |
|---|
C1=C(C=CC2=CC=CC=C12)[C@@]34C[C@@H]3CNC4
|
InChI=1S/C15H15N/c1-2-4-12-7-13(6-5-11(12)3-1)15-8-14(15)9-16-10-15/h1-7,14,16H,8-10H2/t14-,15+/m1/s1 Key:HKHCSWPSUSWGLI-CABCVRRESA-N
|
賽他發定(INN:centanafadine;開發代號:EB-1020)是一種血清素-去甲腎上腺素-多巴胺再攝取抑制劑(也叫三重再攝取抑制劑),在Euthymics Bioscience收購DOV製藥後開始開發。它被開發用於治療注意力缺陷多動障礙,並分別以1:6:14的比例抑制去甲腎上腺素、多巴胺和血清素的再攝取。[1][2][3][4]2011年,Euthymics Bioscience將他們的賽他發定開發業務剝離給了一家名為Neurovance的新公司。[5][6]2017年3月,大冢製藥收購了Neurovance和賽他發定的專利。[7]截至2018年1月,大冢的研發管線顯示其正在進行II期和III期臨床試驗,以應對多種不同的醫療狀況。[8][9][10]
- ^ 1.0 1.1 1.2 1.3 1.4 Neurovance's EB-1020 SR for Adult ADHD Shows Stimulant-Like Efficacy and Good Tolerability in Phase 2a Trial (PDF). Neurovance. [14 January 2018]. (原始內容存檔 (PDF)於2021-11-04).
- ^ 3-Neurotransmitters, 1-Molecule: Optimized Ratios. Neurovance. [2024-06-03]. (原始內容存檔於2022-08-09).
- ^ EB-1020, a Non-Stimulant Norepinephrine and Dopamine - Preferring Reuptake Inhibitor for the Treatment of Adult ADHD (PDF). Neurovance. [2015-11-14]. (原始內容 (PDF)存檔於2015-11-17).
- ^ Bymaster FP, Golembiowska K, Kowalska M, Choi YK, Tarazi FI. Pharmacological characterization of the norepinephrine and dopamine reuptake inhibitor EB-1020: implications for treatment of attention-deficit hyperactivity disorder. Synapse. June 2012, 66 (6): 522–32. PMID 22298359. S2CID 38850652. doi:10.1002/syn.21538.
- ^ Euthymics. Ethismos Research Inc. [14 January 2018]. (原始內容存檔於2024-09-28).
- ^ EUTHYMICS BIOSCIENCE, INC. PRESENTS DATA THAT SUPPORT ADVANCING EB-1020 INTO CLINICAL TRIALS FOR ADULT ADHD (PDF). Neurovance. December 7, 2011 [14 January 2018]. (原始內容存檔 (PDF)於2023-05-29).
- ^ Otsuka Pharmaceutical to Acquire Neurovance, Inc.. Otsuka. [14 January 2018]. (原始內容存檔於2024-06-03).
- ^ Otsuka U.S. Research & Development Programs. Otsuka U.S. Otsuka. [14 January 2018]. [永久失效連結]
- ^ Otsuka Pharmaceutical Development & Commercialization, Inc. A Phase 3, Randomized, Double-blind, Multicenter, Placebo-controlled, Parallel-group Trial Evaluating the Efficacy, Safety, and Tolerability of Centanafadine Sustained-release Tablets in Adults With Attention-deficit/Hyperactivity Disorder. 2021-09-17.
- ^ Gunduz-Bruce, Handan. SAGE-217 in major depressive disorder: a multicenter, randomized, double-blind, Phase 2 placebo-controlled trial. 2018-09-26 [2023-06-26]. S2CID 266120058. doi:10.26226/morressier.5b68175eb56e9b005965c44b.

