窗烷
外觀
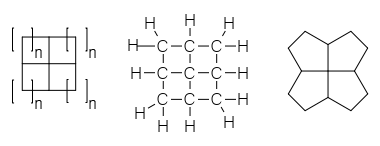
窗烷是一種有機化合物,屬烷烴類,其中心結構是由一個碳原子的四個共價鍵延伸出4個共邊的碳氫環(稠碳氫環)[1],狀如窗戶而得名,可以看成是一類螺烷烴。於1972年由化學家Vlasios Georgian和Martin Saltzman[2]提出,其英文名字Fenestrane來自拉丁文的「窗戶」。
概要
[編輯]正常情況下,中心碳原子的sp3雜化導致其共價鍵呈正四面體結構。窗烷的四環構型則需要這些共價鍵更傾向於平面結構,因此不容易合成。
理論上,最小的窗烷包含4個3員環(環丙烷),稱為[3,3,3,3]窗烷。下一個成員[4,4,4,4]窗烷包含四個稠合的環丁烷體系,如同窗戶一般。
命名窗烷時,只需要將各環中碳原子數目數出,以逗號相隔,並用方括號括起來,後面接上「窗烷」就可以了。窗烷也有系統命名,如[4,4,4,4]窗烷的系統命名是:四環[3.3.1.03,9.07,9]壬烷。
在某些極端情況下正四面體結構的碳徹底變成平面四邊形結構。平面四邊形甲烷的分子軌道圖像表明,三個sp2雜化軌道中的兩個軌道分別與兩個氫原子成普通的σ鍵,第三個雜化軌道與兩個剩餘的氫原子成三中心兩電子鍵,僅利用兩個氫的電子。而碳的兩個未成對電子則被擠進垂直的p軌道。由於共振,四個碳氫鍵是完全等同的。電腦模擬表明完成這樣的過程需要95-250kcal/mol的能量。
張力很大的[4,4,4,5]窗烷已被合成出來。X射線繞射表明中心碳原子鍵角大約為130°,並且鍵長較短(149pm於普通的159pm)。
第一個合成的窗烷是[4,5,5,6]窗烷[3]:

氮雜[4,5,5,5]窗烷也於最近被合成出來,以便結晶被X射線繞射分析[4][5]。

氟硼酸鹽中 N-C-C 鍵角為126°。
另見
[編輯]參考資料
[編輯]- ^ (Eng.)Fenestranes and the flattening of tetrahedral carbon Bhaskar Rao Venepalli and William C. Agosta Chem. Rev.; 1987; 87(2) pp 399 - 410; DOI:10.1021/cr00078a007
- ^ (Eng.)Syntheses directed toward saturated 「flat」 carbon Vlasios Georgian Martin Saltzman Tetrahedron Letters Volume 13, Issue 42 , 1972, Pages 4315-4317 DOI:10.1016/S0040-4039(01)94304-7
- ^ (Eng.)The first step in this reaction sequence is an adaptation of the Stork enamine alkylation reacting cyclopentanone with 3-bromo-1-butene through an imine derivative with pyrrolidine and forming a magnesium salt with ethyl magnesium bromide. The next step is a regular Stork enamine reaction followed by an aldol condensation forming the cyclohexenone ring. The final step is a photolytic [2+2]cycloaddition.
- ^ (Eng.)Synthesis, X-ray Crystallography, and Computational Analysis of 1-Azafenestranes Scott E. Denmark, Justin I. Montgomery, and Laurenz A. Kramps J. Am. Chem. Soc.; 2006; 128(35) pp 11620 - 11630; (Article) DOI:10.1021/ja0632759
- ^ (Eng.)In step 1 the alkyl halide 1-iodo-3-butene 1 is converted to a cyanozinc cuprate 2 (by transmetalation of the organozinc iodide with copper cyanide) which reacts in the next step with 1-nitro-cyclopentene 3 in a nucleophilic addition whereby the nitronate 4 is captured by phenylselenenyl bromide to the selenium intermediate 5. Hydrogen peroxide oxidation of 5 yields the nitroalkene 6 as a mixture of syn and anti isomers. A [4+2]cycloaddition with n-butyl-enol ether in presence of trimethyl aluminum gives the nitronate 7 and a second [3+2]cycloaddition by heating in presence of potassium carbonate gives the nitroso acetal 8. Hydrogenation with Raney nickel gives the diol 9 which on a double Mitsunobu reaction (with an amine proton donor) gives the azafenestrane 10 as the borane salt.
