福山偶聯反應
外觀
Fukuyama偶聯反應(福山偶聯反應)
由日本人福山透(Fukuyama Tōru)等在 1998 年發現。是將羧酸及其衍生物轉變為酮的方法之一。反應選擇性高,有機鋅試劑毒性較小,活性較低,因此反應條件溫和,許多官能團(如醛、酮、氯代芳烴、硫醚等)不受影響。[2] 反應到酮停止,不再繼續將酮還原為醇。
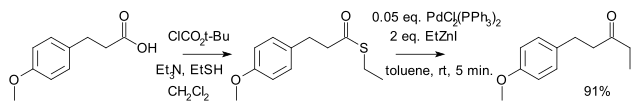
反應機理
[編輯]應用
[編輯]
參見
[編輯]參考資料
[編輯]- ^ Hidetoshi Tokuyama, Satoshi Yokoshima, Tohru Yamashita and Tohru Fukuyama. A novel ketone synthesis by a palladium-catalyzed reaction of thiol esters and organozinc reagents. Tetrahedron Letters. 1998, 39 (20): 3189–3192. doi:10.1016/S0040-4039(98)00456-0.
- ^ Yoshikazu Mori, Masahiko Seki (2007). "Synthesis of Multi-functionalized Ketones through the Fukuyama Coupling Reaction Catalyzed by Pearlman's Catalyst: Preparation of Ethyl 6-Oxotridecanoate". Org. Synth.; Coll. Vol. 84: 285–294.
- ^ Toshiaki Shimizu and Masahiko S. Facile synthesis of (+)-biotin via Fukuyama coupling reaction. Tetrahedron Letters. 2000, 41 (26): 5099–5101. doi:10.1016/S0040-4039(98)00456-0.






