福山偶联反应
外观
Fukuyama偶联反应(福山偶联反应)
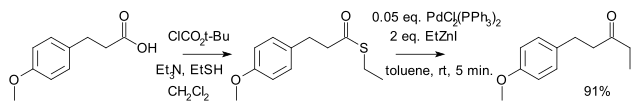
由日本人福山透(Fukuyama Tōru)等在 1998 年发现。是将羧酸及其衍生物转变为酮的方法之一。反应选择性高,有机锌试剂毒性较小,活性较低,因此反应条件温和,许多官能团(如醛、酮、氯代芳烃、硫醚等)不受影响。[2] 反应到酮停止,不再继续将酮还原为醇。
反应机理
[编辑]应用
[编辑]
参见
[编辑]参考资料
[编辑]- ^ Hidetoshi Tokuyama, Satoshi Yokoshima, Tohru Yamashita and Tohru Fukuyama. A novel ketone synthesis by a palladium-catalyzed reaction of thiol esters and organozinc reagents. Tetrahedron Letters. 1998, 39 (20): 3189–3192. doi:10.1016/S0040-4039(98)00456-0.
- ^ Yoshikazu Mori, Masahiko Seki (2007). "Synthesis of Multi-functionalized Ketones through the Fukuyama Coupling Reaction Catalyzed by Pearlman's Catalyst: Preparation of Ethyl 6-Oxotridecanoate". Org. Synth.; Coll. Vol. 84: 285–294.
- ^ Toshiaki Shimizu and Masahiko S. Facile synthesis of (+)-biotin via Fukuyama coupling reaction. Tetrahedron Letters. 2000, 41 (26): 5099–5101. doi:10.1016/S0040-4039(98)00456-0.






